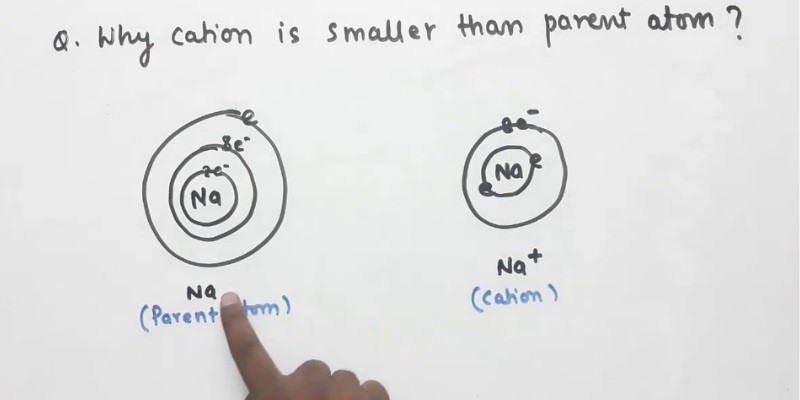
Cations are atoms that have lost one or more electrons and have a net positive charge. The size of a cation is determined by the number of protons in its nucleus. The number of electrons lost has no effect on the size of the cation.
The size of an atom is determined by the size of its nucleus. The size of the nucleus is determined by the number of protons it contains. The number of protons in the nucleus determines the strength of the positive charge.
The more protons in the nucleus, the stronger the positive charge and the smaller the atom. Cations are atoms that have lost one or more electrons. When an atom loses an electron, the nucleus becomes more positively charged.
The more electrons an atom loses, the more positive the charge and the smaller the atom.
One of the most common questions that students ask about atoms is why cations are smaller than their parent atoms. The answer to this question lies in the way that atoms form cations. When an atom forms a cation, it loses one or more electrons from its outermost orbital.
This loss of electrons causes the atom to become positively charged, and the positively charged nucleus is drawn closer to the electrons that are still in orbit around it. This closeness causes the atom to become smaller. It’s important to remember that not all atoms form cations in the same way.
Some atoms lose more electrons than others, and this affects the size of the resulting cation. Additionally, some atoms have larger nuclei than others, which also affects the size of the resulting cation. In general, though, cations are smaller than their parent atoms because of the way that atoms form cations.
The loss of electrons causes the positively charged nucleus to be drawn closer to the electrons that are still in orbit around it, and this closeness causes the atom to become smaller.
Why cation is smaller that parent atom? Class 11- periodic table
Cations are always than the parent atom
When it comes to atoms, size really does matter – at least when it comes to their charge. Cations, or positively-charged ions, are always smaller than the parent atom. This is because when an atom loses electrons, it also loses some of its size.
The smaller the atom, the greater the charge. This is why cations are so important in chemistry. They can help balance out the charges of molecules and enable reactions to occur.
In fact, most of the chemical reactions that occur in the world involve cations in some way. So next time you see a cation, remember that it’s not just a big deal, it’s a small one too.

Credit: www.toppr.com
Why are cations smaller than their parent atoms?
Cations are atoms that have lost one or more electrons, resulting in a net positive charge. The reason cations are smaller than their parent atoms is because they have lost electrons, which are negatively charged. The loss of electrons results in a smaller atom because electrons take up space.
The smaller size of the cation allows it to fit into smaller spaces between atoms, which is why cations are often found in minerals.
How does this impact the way they behave?
There are many different ways in which people can be impacted by their environment. The environment can affect people in both positive and negative ways, depending on the circumstances. For example, if someone grows up in a safe and nurturing environment, they are more likely to develop into a well-adjusted and emotionally stable individual.
However, if someone grows up in an environment that is neglectful or abusive, they are more likely to develop into an individual who struggles with mental health issues and/or behavioral problems. There are many different factors that can contribute to someone’s environment, such as their family life, their socioeconomic status, their community, and so on. All of these factors can play a role in shaping a person’s behavior.
For instance, someone who comes from a wealthy family is more likely to have access to resources and opportunities that can help them succeed in life. On the other hand, someone who comes from a poverty-stricken background is more likely to struggle in life and may turn to negative coping mechanisms, such as drug use, in order to deal with their circumstances. It’s important to remember that everyone is different and that not everyone will be impacted in the same way by their environment.
Some people are able to overcome negative environmental influences and go on to lead happy and successful lives. However, for others, their environment can be a major contributing factor to their struggles. If you or someone you know is struggling, it’s important to seek out professional help in order to address the underlying issues.
What implications does this have for chemical reactions?
In a chemical reaction, atoms are rearranged to form new molecules. The amount of energy required to break the bonds between the atoms is called the activation energy. The amount of energy released when the new bonds are formed is called the heat of reaction.
The heat of reaction can be used to calculate the change in enthalpy, which is a measure of the amount of energy required to break the bonds between the atoms. The enthalpy of a reaction is the heat of reaction divided by the number of molecules of reactants. The activation energy is the energy required to break the bonds between the atoms.
The heat of reaction is the energy released when the new bonds are formed. The change in enthalpy is the heat of reaction divided by the number of molecules of reactants.
Conclusion
Cations are atoms that have lost one or more electrons, and as a result, have a positive charge. Because they have fewer electrons, cations are smaller than their parent atoms. This is due to the fact that electrons are attracted to the nucleus, and the more electrons an atom has, the greater the attraction.
When an atom loses an electron, the attraction between the nucleus and the remaining electrons is reduced, and the atom becomes smaller.
Last Updated on July 28, 2022 by Marjorie R. Rogers, MA (English), Certified Consultant